Module 6: Lesson 5
Self-Check 3
- Question 16 on page 402 of the textbook
-
- To solve this question, the formula pH = −log[H+] should be used.
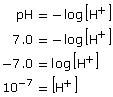
Thus, the hydrogen ion concentration in a patient with a pH of 7.0 is 10−7 mol/L.
- The formula that was used in Try This 4 can be used here.
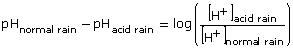
Substituting the known values gives the following:
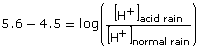
Converting from logarithmic form to exponential form will allow the answer to be solved.
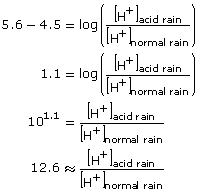
Thus, acid rain with a pH of 4.5 is approximately 12.6 times as acidic as normal rain with a pH of 5.6. - The formula that was used in Try This 4 can be used here.
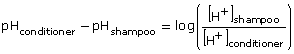
Substitute the known values into the equation.
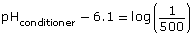
Solve for the unknown value.
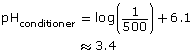
Thus, the pH of the conditioner is approximately 3.4.
- To solve this question, the formula pH = −log[H+] should be used.
-
© 2012 Alberta Education