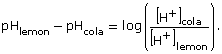You have practised using the laws of logarithms to simplify expressions. You can also use the laws to help you simplify problems. In Try This 4 you will use what you have learned to solve a problem with a logarithmic scale.
Try This 4
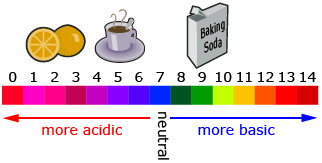
The pH scale is a logarithmic scale that is used to communicate the acidity or alkalinity of a solution. To determine the pH of a solution, the equation used is pH = − log [H+], where [H+] is the concentration of hydrogen ions measured in moles per litre (mol/L). The square brackets around [H+] indicate concentration. Refer to the image to see pH values for some common substances. Also make note of the pH values that describe acidic, neutral, and basic substances.
- What would be the pH of vinegar that has the following concentration of hydrogen ions: [H+] = 0.006 31 mol/L.

- Coffee has a pH of 5.4. How many times as acidic as coffee is vinegar?

- A lemon-flavoured pop has a pH of 2.8. A cola-flavoured pop is 3 times as acidic as the lemon-flavoured pop. What is the pH of the cola-flavoured pop?

![]() Save your responses in your course folder.
Save your responses in your course folder.
Logarithmic scales are useful when you have a very large range of values. In the pH scale, the concentration of hydrogen ions has a very large range between solutions. Many logarithmic scales are base 10, so this means that an increase of 1 on the scale is actually a factor of 10. For example, a pH of 4 is 10 times more acidic than a pH of 5.