In Try This 2 you examined the graphs of different exponential functions. You determined characteristics of each function from the function’s graph. In Try This 3 you will look at determining the equation of an exponential function when given the function’s graph.
Try This 3
The medical isotope Iodine-131 is produced at Chalk River Laboratories in Ontario. The isotope is used in nuclear medicine. A radioactive sample of Iodine-131 has a half-life of 8 d. This means that after eight days, half of the original amount of the sample has decayed. The original sample is 1.0 g. The graph of the amount of Iodine, A, remaining over time, t, in 8-d intervals is shown.
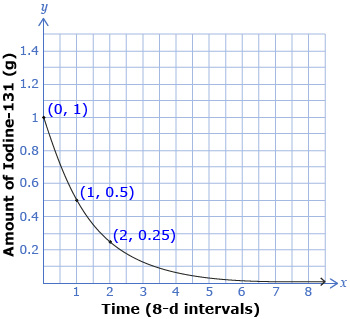
- What are the domain and range of this graph?
- Based on the shape of the graph, is c > 1 or 0 < c < 1?
- Place the three data points in a table like the one that follows. As the x-value increases by 1, by what factor does the y-value change? This factor describes the pattern of decay.
x
y
- Use your answer from question 3 as the c-value in an exponential function that would model the relationship between the amount of Iodine-131, A, and time, t, in 8-d intervals.
- To verify your function from question 4, substitute the point (2, 0.25) into your function. Is your function correct?
- Estimate how many days it would take for there to be 0.0625 g of Iodine-131 remaining. Explain how you determined your answer.
![]() Save your responses in your course folder.
Save your responses in your course folder.

© Photo courtesy of the National Research Council
The National Research Universal Reactor produces the following isotopes:
- Molybdenum-99: used for medical diagnosis of the brain, thyroid, heart, lungs, liver, kidney, spleen, and bone marrow
- Iodine-131: used in therapy, imaging, and diagnosis, primarily for the thyroid
- Iodine-125: used in in-vitro diagnostics, bone densitometry devices, protein iodination, and therapeutic seed
- Xenon-133: a medical diagnosis tool, especially for scanning lungs1
1 Adapted from Medical Isotopes/Atomic Energy of Canada Limited